Abstract
Background: Outcomes of patients with complex karyotype (CK) and TP53-mutated acute myeloid leukemia (AML) are poor. Many centers use '7+3' as upfront therapy in patients fit to receive intensive chemotherapy. Venetoclax with hypomethylating agent (HMA/Ven), CPX-351 and various high dose cytarabine containing regimens are also used as front-line therapy in this high-risk population, with variable results. The optimal induction regimen remains unclear. Furthermore, there is a hesitancy to consolidate with allogeneic stem cell transplantation (allo-SCT) in this population because of the high risk of post-transplant relapse.
Methods: We conducted a retrospective analysis to investigate the impact of various induction regimens, along with consolidation with allo-SCT, on the outcomes of patients with both CK and TP53-mutated AML treated at the University of Alabama at Birmingham (UAB) between January 2013 and May 2020.
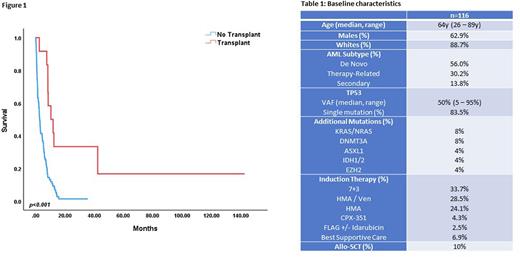
Results: We identified 116 patients with CK/TP53 AML (Table 1). The median age at diagnosis was 64y (26-89y) and majority of the patients were male (62.9%) and white (88.7%). Fifty-six percent of the cases were de novo, 30.2% were therapy-related and 13.8% were other secondary AML. The median variant allelic frequency (VAF) of TP53 was 50% (5-95%) and 83.5% were single mutations. The most commonly occurring co-mutations were KRAS/NRAS (8%), DNMT3A (8%), ASXL1 (4%), IDH1/2 (4%) and EZH2 (4%).
Upfront treatment consisted of 7+3 (33.7%), HMA/Ven (28.5%), HMA (24.1%), CPX-351 (4.3%), FLAG+/-ida (2.5%) and best supportive care (6.9%). The complete remission (CR) rate (including CR with incomplete count recovery) was 19.8% for the entire cohort; 10% of the population proceeded to allo-SCT. The median overall survival (mOS) for the entire cohort was 3.1m. The mOS of patients proceeding to allo-SCT was 10.4m compared to 2.6m for those unable to proceed to transplant (p<0.001) (Figure 1). A subset analysis was conducted to compare outcomes of 7+3 (n=39), HMA/Ven (n=33) and HMA (n=28). The median age for patients receiving 7+3, HMA/Ven and HMA was 58y, 70y and 65y, respectively (p<0.001). There were more patients with de novo AML in the 7+3 arm (79.5%), compared to HMA/Ven (60.6%) and HMA (28.5%) (P<0.001) arms. The CR/CRi rate for 7+3, HMA/Ven and HMA was 10.3%, 39.6% and 7.1% (p=0.003). The proportion of patients proceeding to allo-SCT with 7+3, HMA/Ven and HMA was 15.4%, 12.1% and 3.5% (p=0.06). The mOS for patients receiving 7+3, HMA/Ven and HMA was 2.8m, 5.3m and 3.1m, respectively (p=0.8).
One out of 5 patients in the CPX-351 arm and 2 out of 3 in the FLAG+/-ida arm achieved CR. Among the 35 non-responders to 7+3, 9 were unable to receive any subsequent therapy and proceeded with comfort care only. Ten patients received re-induction with a high dose cytarabine based regimen (6 achieved CR), 10 received HMA/Ven (4 achieved CR) and 6 received 5+2 (2 achieved CR).
Twelve patients proceeded to allo-SCT. Six received 7+3 as first line therapy, 4 received HMA/Ven, 1 received FLAG and one received HMA. All 6 patients that received either HMA/Ven, FLAG or HMA, went into CR with first line therapy. For the 6 patients that received 7+3, only 1 went into CR. For the remaining 5 patients, 2 achieved CR with FLAG, 2 achieved CR with HMA/Ven and 1 achieved CR with 5+2. Out of the 12 patients that proceeded to allo-SCT, 5 relapsed within the first year.
Conclusion: Outcomes of patients with CK/TP53 AML remain poor. Our results suggest that 7+3 is not the ideal induction regimen and perhaps HMA/Ven may be a better option to induce remission. Overall outcomes still remain dismal for these patients. Transplant can prolong survival for a subset of patients; however, risk of post-transplant relapse is high and strategies to mitigate this risk are needed.
Disclosures
Vachhani:Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma Corp: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal